

Clean room systems are systems used in industrial and scientific studies, where humidity, temperature, pressure, air flow in the environment, particulate pollution in the environment and possible contamination factors are controlled and controlled. ISO 14644, on the other hand, is a family of standards that includes 18 sub-standards that enable the installation, classification, implementation, testing and inspection of clean room systems. Clean room according to ISO 14644-1; For example; It is defined as a room in which temperature, humidity and pressure are properly controlled.

Along with the clean room systems used, manufacturers who adhere to GMP requirements in pharmaceutical production, medical device production, laboratories and similar fields are obliged to meet the requirements of clean room systems. All requirements are specified in ISO 14644 with 18 subsections. These sections are shown in Table 2. Thanks to these standards, the quality control and reliability of the produced product are ensured.
Classification in Cleanroom
According to the ISO 14644-1 standard definition, it is known as the classification of air cleanliness by particle concentration. This section specifies the standard test method and reporting contents for determining the air cleanliness class of the room in terms of particle concentration in the air volume. ISO air cleanliness classes according to particle concentration are shown in Table 1.
Table 1. ISO Air Cleanliness Classes by Particle Concentration

According to Table 1., ISO 1 class rooms show the highest cleaning level, while ISO 9 class indicates the lowest cleaning level. In addition, even the most polluted ISO 9 class offers a cleaner environment than a normal room. The appropriate ISO class for the product produced by the manufacturer must be provided. In this way, the presence of particles that may endanger the sterility and quality of sensitive products will be minimized. Before entering the production work area, the personnel must ensure aseptic conditions such as bonnets, masks, tops, shoe covers and gloves in the locker room.
According to the ISO 14644-4 standard, a project plan should be created in consultation with all relevant parties to define the requirements of the products, processes and scope of installation during the planning phase of a cleanroom. Requirements, equipment list and critical requirements for each equipment should be determined.
Validation of the clean room
ISO 14644-1 and ISO 14644-3 standards are used to validate cleanroom systems. According to the tests determined by this standard, qualification is made by obtaining the result of whether the cleanroom is valid or not. All devices to be used during the test must have up-to-date calibration certificates and the personnel who will perform the test must be certified. Validation and qualification should be done at least once a year in cleanrooms. According to ISO 14644-2; prior to particle counting in the evaluation of ventilation systems;
It should be measured, then particle measurement should be made and ISO class of the cleanroom should be determined according to the particle measurement. The ISO 14644 standard considers the possibility of testing Clean Rooms under three different conditions;
First finished: in this scenario, operators take measurements when the clean room is finished, without machinery and personnel;
At standstill: In this case, measurements will be taken while the machine is in operation without personnel;
In operation: In this case, measurements should be made with machines and personnel present and normally active.
Validation tests consist of 8 different stages.
If the cleanroom is done correctly to standards, the results of the validations will be just as accurate. For this reason, it is very important that companies that produce in clean rooms are companies that know the standards well and are specialized in this field.
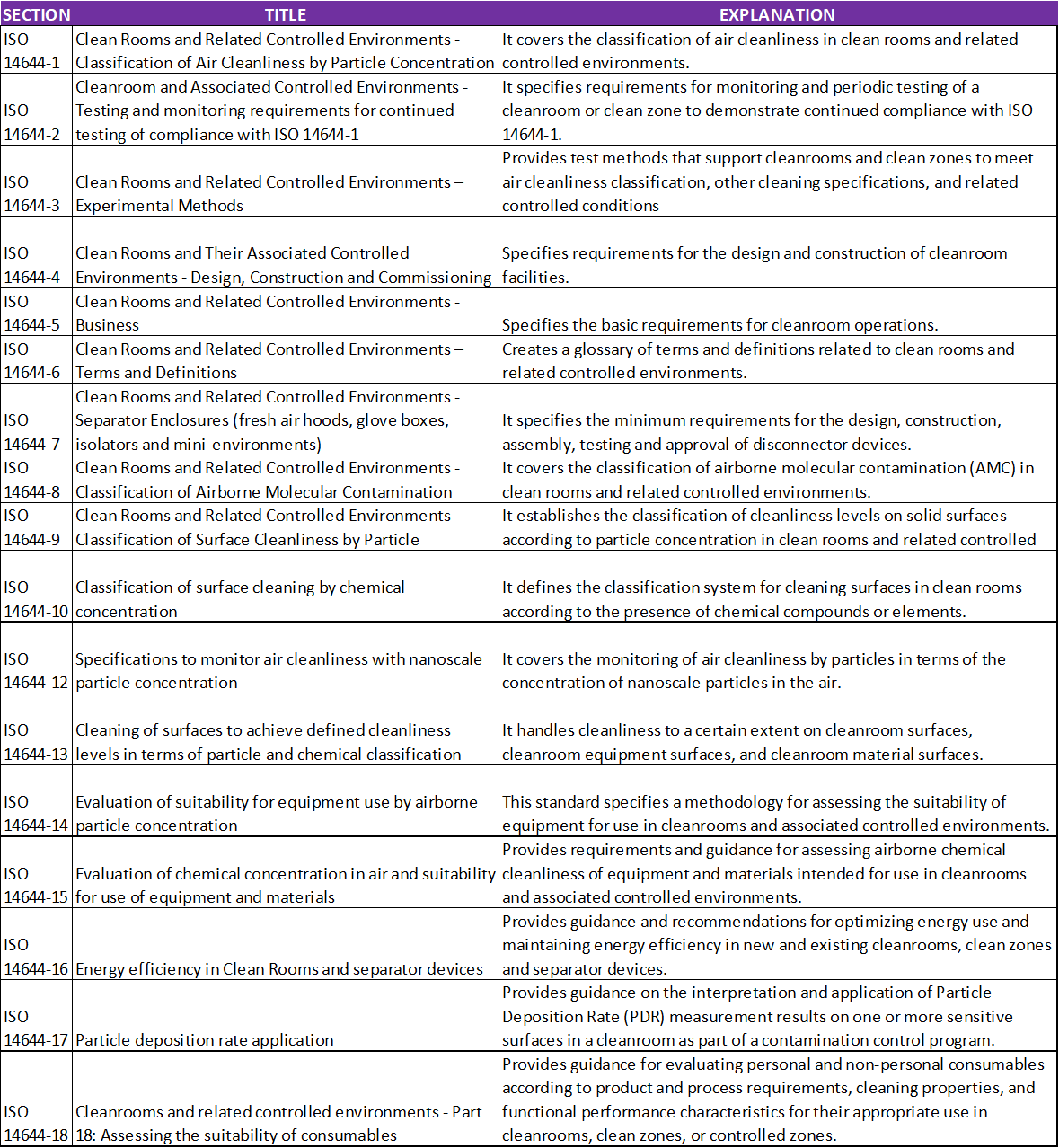
ISO 14644 Standard Family
Table 2. ISO 14644 Standard Family
You stumbled upon this article by chance and couldn't decide whether your planned application might fall under the MDR or IVDR? Are you new to the industry? Do not hesitate, experienced Infigen Consultancy, which has been dealing with certification processes for 20 years, can assist you in your studies.
Please note that all details and information from Infigen Consultancy do not claim to be complete and are for informational purposes only.
